What is refinement? Investigate the refinement of a base material towards the creation of a refined product. My starting material had to be wood. However, coppiced wood has been a draw to me for awhile due to its totally sustainable and low-impact cycle.
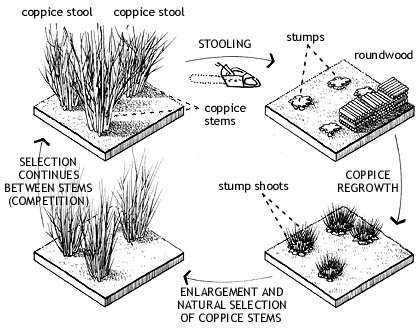
Coppicing is a tradition of woodland management; working to a cycle of growth of trees which have been sensitively selected and felled for regrowth. Trees have dormant buds lying in wait under their bark in defense to damage, like getting munched by Mammoths or stamped on by any other significant mammal. As the network of roots remain, replacement growth happens considerably faster than for a new tree to grow from seed. For centuries humans have manipulated this natural trait to provide an abundant supply of raw material. After every harvest, a tree stool will provide, on average, 3 times the number of new shoots. Historically, coppiced areas are left for 5-7 years to regenerate and produce quick grown, straight poles, prime for the greenwood craft industry.
Essentially, coppice is a sustainable and truely renewable source of carbon-neutral material. Whats-more, its potential is everywhere. There are around half a million acres of unmanaged woodlands in the UK. To see a new wave of industry utilising this resource for our markets and manufacturing would be a huge step forward for the conservation of our countryside.
The draw back in working with such a material is that it's inherent beauty and purity, it's organic-ness makes it difficult to take coppiced / green wood forward as a realistic contender for batch production. To work with it is labour-some and time consuming, if not for stunning results. Now, how can I make afford products with this environmentally affordable material?
Of all the green wood craft, I am most intrigued by Swill Baskets. The principle is taking green Oak, granted as straight as possible, and boiling it for at least 24 hours allowing it to be split to fine strips. Essentially the wood is being made into a generic form of itself, to be flexed and weaved into a basket. As it dries the wood hardens and the basket becomes incredibly strong.
It is the reaction of the wood to the boiling that has intrigued me. What happens to the make-up of the wood to make it go pliable when wet, and retain shape and rigidity when it dries?
To understand wood a little better, on a microscopic scale I got in touch with a research scientist from Innovia Films. They produce cellophane by the tonne. Cellophane is pure cellulose extracted from wood pulp. The process of extraction is fairly similar to paper making. Huge quantities of wood pulp are re-boiled in a water and Sodium Hydroxide solution; a soup that speeds up the separation of cellulose from the 2 other essential ingredients in wood (The Kraft process).
Now here's the bio-chemistry bit. Wood is made up of cellulose, hemi-cellulose and lignin.
 Cellulose is the structure of the wood. The molecular fibres which act together to create rigidity.
Cellulose is the structure of the wood. The molecular fibres which act together to create rigidity.
Hemi-cellulose is the 'string' that holds the cellulose fibres together.
This knot of cellulose is set in lignin. The 'resin' which glues the structural molecules together.
When the cellophane and paper makers, as well as swill basket makers, boil their wood they are diluting the lignin. In the example of boiling and steaming wood in the wood craft industry, the dilution of the lignin allows the wood to be bent and formed into new directions. As the water evaporates and the lignin condenses, the wood stiffens and retains its new shape.
From this finding I have been developing an interest in Lignin and what it's properties could be used for. Although a waste product from these different techniques, it has a great calorific value, it has more carbon content than wood itself. The Kraft process burns the recovered lignin to recycle its energy back into the system.
Understanding these two comparably different scales of wood manipulation, I started to wonder if the use of Sodium Hydroxide (Caustic soda) in the industrialisation of cellophane was completely necessary. Reading into the science of woods molecular bonds I believe a high pressure and high temperature hydrolysis method would be enough.
First step: Try to cook and separate wood at home with a Pressure Cooker.
The smaller the carrots the quicker the cooking, right!? I reused the brown water from the previous cook. I figured I'd intensify the concerntation.
Although the sight of a pressure cooker steaming away on the stove was a regular occurance during my childhood, I have no idea how to use one, especially when cooking wood for 6 hours. The water levels were calculated wrong and that was the end of this test.
These tests were an interesting starting point. I've been left with some interesting brown liquour. Somewhere, floating in this milk bottle is weaker bonded lignin. The wood has barely changed, so the cooking process definitely needs taken up a knotch or two.
To the laboratory...